- People under the age of 30 should not be offered the AstraZeneca vaccine, UK regulators have said.
- The news comes as cases of unusual blood clots after vaccination were being reviewed.
- Risk of rare adverse events remain "extremely low", but risk to benefit is "more finely balanced" in younger populations.
- See more stories on Insider's business page.
The AstraZeneca coronavirus vaccine should not be given to people under the age of 30, UK regulators said Wednesday.
The news was reported by the Guardian and MailOnline just before a press conference by UK government officials. Selected media had been briefed on the conclusion in advance.
The Guardian reported that the UK's Joint Committee on Vaccines and Immunisation (JCVI) recommended offering under-30s another vaccine instead.
Most people of that age have not yet been offered vaccines in the UK, which has concentrated on older demographics first.
The reason for the change is a small number of blood clots in people given the vaccine. Officials at the briefing described them as a "suspected" side-effect of the shot, and said that it was very rare.
At the briefing Wednesday, Jonathan Van-Tam, the Deputy Chief Medical Officer for England, described the change as a "course correction" and said that the vaccine rollout had overall been a success.
Dr June Raine, Chief Executive of Medicines and Healthcare products Regulatory Agency (MHRA) said of the blood clots that "the risk of this rare of this rare suspected side effect remains extremely small."
The logic of restricting the vaccine from use in younger demographics is that they are less at risk of COVID-19, and slightly more at risk from the clots, officials said at the briefing.
This chart from the MHRA was shown to describe the balance of risks:
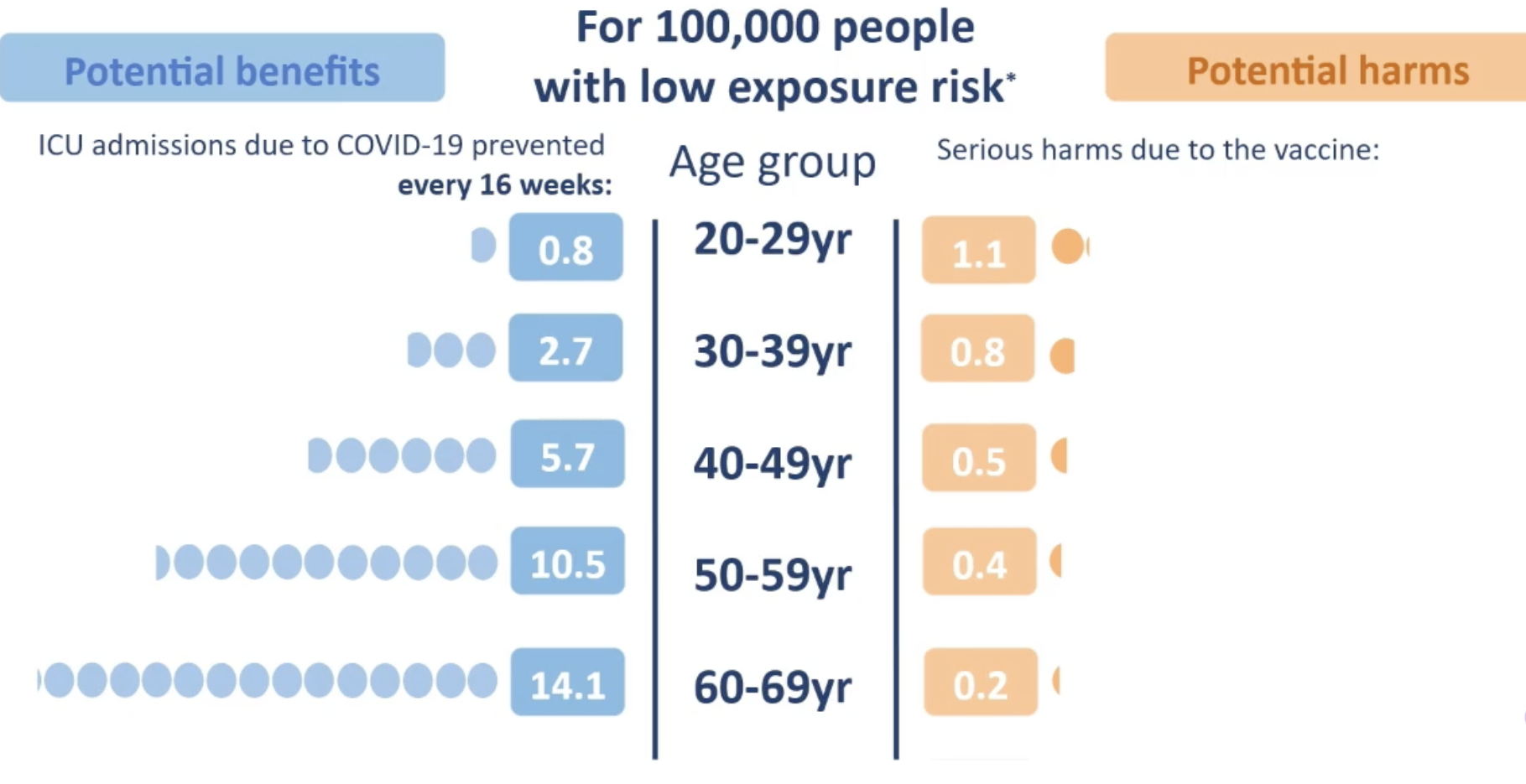
MHRA
Similar reports of blood clots prompted earlier suspensions of the AstraZeneca rollout in many countries, some of which later restricted the use of the shot in younger demographics.